Bomb Calorimeter: Definition, Construction, Diagram, Working & Uses
Definition of Bomb Calorimeter
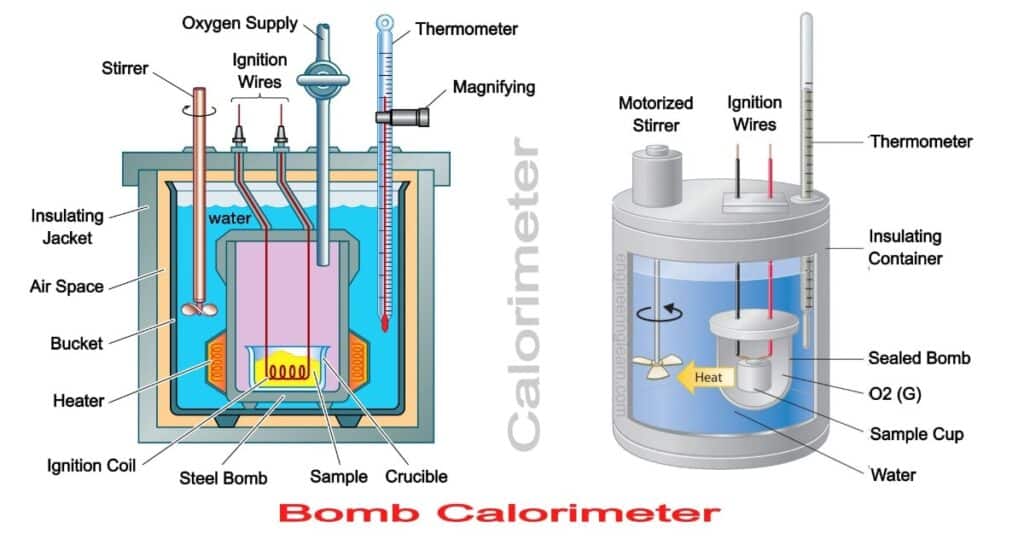
Bomb Calorimeter: Definition, Diagram, Working & Uses :- Bomb calorimeter is referred to as that calorimeter which is mostly used in order to determine the change of energy which is caused during a reaction accurately. The modern Bomb calorimeter is known to be a developmental part of the original calorimeter of Berthelot. The modern bomb calorimeter is found being made of corrosion resistant steel which includes the combination of the Bomb Calorimeter.
The bomb calorimeter is referred to as an instrument which is mostly used for the purpose of measuring the heat of reaction that is found having a fixed volume in order to measure the heat which is also termed as the change of internal energy (ΔE). Chemically, the changes of heat reaction needs to be measured at fixed pressure or volume.
Construction of Bomb Calorimeter
The bomb calorimeter is found having properties which help in determining the calorific values of solid as well as liquid fuels. Bomb calorimeter is usually an instrument which is found consisting of a strong steel shell that is commonly known as a bomb.
These are found consisting of a base which is likely to support the platinum crucible and is also found to be screwed within the body of the bomb. The top portion of the bomb is responsible for carrying an oxygen supply connection accompanied with a valve in order to release the product.
For instance, if there is a one gram of powdered sample coal which is taken for the test and the calorimeter is filled with 2000cm3 of water. The sample needs to be placed in the platinum crucible for this purpose. The iron fuse wire is found to surround the sample of coal which is found being connected to the lower end of any two electrodes. Usually, the electrodes are responsible for extending it through the base of the bomb which needs to connect the fuse wire so that an electric circuit can be ignited by closing the complete electric circuit.
Construction of Bomb Calorimeter
The bomb seems to be placed within the copper vessel which is found containing water. There is a noticeable stirring device which is used for agitating the water within the calorimeter. The calorimeter is responsible for containing the bomb which is placed in any other container that needs to act as a heat insulator. The temperature of water also needs to be monitored within the calorimeter which is done by using a thermometer.
The fuel is observed to ignite by passing a current using the fuse wire. The temperature of both of them seems to start increasing and meanwhile the readings on the thermometer are also found to be taken at an equivalent interval of one minute for 10 consequent minutes. Once the maximum intervals for 10 minutes is completed the maximum temperature is also observed to be reached. After this also the temperature starts falling but the process is observed to be quite slow. Whenever there is a fall in the temperature, a steady rate of the readings are taken at consistently regular intervals for an additional five minutes.
The addition of amount of heat given by the combustion of coal and heat given by the combustion of fuse wire is equivalent to the heat taken by the water and calorimeter.
Working of Bomb Calorimeter
The bomb calorimeter is known to be a type of constant-volume calorimeter which is usually used in order to measure the combustion heat of oxygen-burnable samples. There are four critical parts which are mostly needed for almost every bomb calorimeter.
The bomb calorimeter is known as a laboratory instrument which is commonly used in order to measure the amount combustion heat or heat power of a sample whenever there is an excess oxygen combustion found to occur. The motive of such types of research is to determine the impact of using a bomb calorimeter along with the ability of physics in order to process science. There are influences which involve the efficacy of using the devices to make you learn how to develop the abilities of the scientific method of students before as well as after using the materials.
If the heating capacity of the calorimeter Coal of any calorimeter is found being known, then you can definitely determine the amount of heat which is generated by the only needs in order to note the change in the temperature process. The usage of calorimeter is widely done in different types of laboratories in today’s time.
Uses of Bomb Calorimeter
A bomb calorimeter is referred to as an instrument which is usually used for the purpose of determining the amount of heat which seems to be emitted from a given quantity of biomass sample combustion in order to calculate the HHV of that particular biomass fuel. Approximately one gram of sample fuel is observed to be ground or diluted after each test in order to fit it into a capsule for bomb combustion. The amount of heat emitted is responsible for increasing the temperature of the water which needs to cover the bomb by the process of combusting the fuel. The total amount of heat of the fuel can be determined by directly increasing the temperature as well as the real mass of the fuel.
There are different industries as well as academic environments for which there seems to be different calorimeters which are found to be helpful. For the same, the industrial pilot plant can be used as a DSC in order to assess a shift in the formula of a substance and to also see what its impact at the formula are. In order to calculate the amount of heat in food, most commonly an oxygen bomb calorimeter is useful for testing the food in the laboratories.
1. Educational Training
This is found to be another common use of a bomb calorimeter which is usually done in the education training. Calorimetry is referred to as a technique which is taught in various university-level courses and also in some of the high school classes. All those individuals which are found pursuing careers in this field are found requiring the use of bomb calorimeter which is found to be must and has become very familiar with the processes that are involved by with using a bomb calorimeter.
2. Thermodynamic Studies
The technology of bomb calorimeter is known to be one of the most basic form of scientific study of to understand the thermodynamic processes. A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced in a chemical reaction. This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. The bomb calorimeters are found to be quite essential in order to complete the scientific as well as theoretical thermodynamic studies.
3. Metabolic Studies
Bomb calorimetry is a technique which can easily be used for the purpose of determining the calorie content of a product. This is known as a process which is commonly used in food as well as in metabolic studies in order to examine the effects of energy content in the food as well as in humans and animals. There are studies which have implications that need to extend into the nutritional considerations as well as health concerns regarding the effects of diet on the body.
4. Propellant and Explosive Testing
Propellants and explosives also need to be tested which can be done using a bomb calorimeter in order to find the heat of detonation of each of them. The propellants are usually the ones which typically burn predictably that too at a very steady rate whereas the explosives are found to be unstable which on the other hand are found exerting a huge amount of pressure accompanied with the induction of the chemical reaction about which both of these processes are identified using the instrument bomb calorimeter.
5. Fuel Testing
Bomb calorimeters are the instruments which are also used in order to test the calorific value of solid as well as liquid fuels that seem to be traded on the base of that particular value. The commonly known fuels like the coal and oil are the ones which are must and should meet the regulations that can specify the total amount of calorific value, quality as well as the purity of the fuel. All the liquid fuels like gasoline and kerosene need to be tested by using the bomb calorimeter. Measuring the energy give-off by the fuel is found to be determined by the fuel’s heat of combustion.
6. Waste and Refuse Disposal
The cement industry is one amongst various other industries which mostly uses the hazardous waste as an alternative fuel. Whereas, the use of such hazardous waste as a fuel seems to be regulated by the government that can include the Environmental Protection Agency (EPA). The instrument bomb calorimeter has an application here, where this simply is needs in order to determine whether the hazardous waste fuel can meet those regulations or whether it is safe and appropriate for the use or not.
Image Source :- thoughtco,